Version: 2020 (see DTU Learn for current year)
The
current year's course plan is communicated via
DTU Learn only. The 2020 list here is kept for reference so participants can "look ahead", although changes will happen.
Student
presentations
are followed-up by Lars' later lectures that also contain new material. The
student presentations are not part of the exam curriculum whereas slides from the follow-up lectures are.
In the reading list below, "Nishimura" is short for "Principles of Magnetic
Resonance Imaging by Dwight Nishimura" (the 2010 and 2016 editions are similar).
The specified pages are for everybody
to read, whereas additional material is sometimes specified for the
student presenters as inspiration (it is up to them to decide how they
will cover the specified topics). For some topics, Nishimura
is supplemented with other obligatory material.
The exercises specified below (except report assigments) are not obligatory, and should not be
handed in. The exercises themselves are not
discussed at the exam, but the methods employed for
these may be (including math).
- Course week 1, 6/2-2020.
- Course week 2, 13/2-2020. Marie and Bianca present.
- Course week 3, 20/2-2020. Hanne and Christine present.
- Course week 4, 27/2-2020. Michelle and Mark present.
- Course week 5, 5/3-2020. Mikkel and Anders present.
- Tuesday March 10th at noon: Obligatory hand-in via CampusNet of assignment on T1 and relative PD estimation from 3D FLASH.
- Course week 6, 12/3-2020. Student presentation postponed.
Informal "midway evaluation" around here.
- Course week 7, 19/3-2020. Isabelle and Steen present.
- Course week 8, 26/3-2020. Anna and Sidsel present.
- Course week 9, 2/4-2020. Wenjun presents.
- Easter.
- Course week 10, 16/4-2020.
- Tuesday April 21st at noon: Hand-in via CampusNet of spectroscopy assignment.
- Course week 11, 23/4-2020.
- Sunday 26/4-2020 at noon: Hand in of revised T1/PD assignment.
- Course week 12, 30/4-2020.
- Course week 13, 7/5-2020.
- Saturday 9/5-2020 at 6 pm: Hand in of revised spectroscopy assignment.
- Friday 15/5-2020: Exam
Introduction lecture:
- Bloch equations in absence of RF: Relaxation and the frame of reference rotating at the Larmor frequency. Nishimura p. 45-53, 57-60.
- Introduction to the course and to practical exercises. See homepage.
You may benefit from visualizing the situations below using the
Bloch Simulator, but the plotting should be performed in Matlab.
- For the first exercise you need to solve the equation of motion of the magnetization for a static magnetic field as done in the lecture, but with inclusion of the relaxation terms instead of the precession term.
Starting from equilibrium, and following 90 degree excitation, calculate analytically the evolution of the longitudinal and transversal magnetization components Mz and |Mxy| for a substance characterized by properties T1, T2, M0 (proportional to the proton density PD).
- The excitation is followed by sampling of the MR signal at time TE after excitation (the echo time). Normally, the signal is sampled for a short period around TE, but here we consider situations where a single sample is acquired at time TE after the excitation.
As function of TE, plot the transversal and longitudinal magnetization of CSF, white matter and gray matter at 1.5T (see Nishimura p. 52. Use relaxation times T1=4s and T2=2s for CSF though these are only approximate). The magnetization can conveniently be given in units of M0
for CSF (i.e. PD=1 for this substance in accordance with relative spin densities given on p.52 in Nishimura).
- We now consider repeated excitation where the sequence above is repeated after a time TR following the first excitation (the repetition time). Assume Mxy=0 before the second pulse, i.e. TR>>T2 (consider why). Calculate how the magnetization evolves after the second excitation. Plot the signal as a function of TE again, but for the second signal readout. Explain the difference to the first curve.
-
For the second signal readout, plot the signal for TE=10 ms as a function of TR. Explain the curve.
- Consider again the sequence with just a single 90-degree excitation. Add a 180 degree inversion pulse to the beginning, and a subsequent delay TI before the excitation pulse (making it an inversion recovery sequence). Calculate the inversion time TI needed to suppress the CSF signal. Calculate the effect of the inversion pulse on the signal of the other tissues.
Lecture:
- Saturation. FLASH. Effect on weighting of echo time (TE),
repetition time (TR), tip angle , inversion time (TI), Nishimura 7.4 p. 146-154.
- MRI safety: Bioeffects, heating effects, projectiles,
precautions, controlled modes, contra-indications, legislation.
Marie and Bianca present:
- Bloch equations for static B0 field and static B1-field on and
off resonance. The effective field vector. Dynamics in the reference
frame rotating at the RF-frequency. Nishimura chapter 6, p. 99-107.
Last week you calculated signal equations. As described below, you will now use the important signal equation for the FLASH sequence to calculate artificial MR-images corresponding to particular tissue parameters. These images will be useful for validating your software developed later for analysis of real brain scans.
- Load tissue masks for CSF, white and gray matter, located at CampusNet/ExerciseData (32 bit floating point, 217x181). Calculate artificial FLASH images corresponding to those in week 2 lecture notes (TR=100ms, tip angle 10, 20, 30, 40, 50 and 70 degrees, echo time much shorter than T2*). Compare to lecture notes.
- to prepare you for the practical exercise, answer the questions in the detailed description.
- Continue exercise from week 1, and consider changes when tip angles are different from 90 degrees.
Follow-up lecture:
-
Bloch equations for static B0 field and static B1-field on and off resonance. The effective field vector. Dynamics in the reference frame rotating at the RF-frequency. Nishimura chapter 6, p. 99-107.
Hanne and Christine present:
- Spectroscopy, MR-visible nuclei, sensitivity, localization, proton spectra and visible metabolites, echo-time effects. Nishimura p. 53-55. Spectroscopy material on CampusNet.
This excersise is somewhat opposite of the last one, i.e. measurement of contrast parameters (T1,PD) based on images aquired with different acquisition parameters. You will later use the developed software for analysis of data collected during the data acquisition exercise.
- Using artificial MR images from last weeks exercise, develop and validate software that estimates relative proton densities and relaxation times for FLASH images acquired with a range of different flip angles (fitting to the signal equation, using f.ex. Matlab's non-linear data fitting, http://www.mathworks.se/support/tech-notes/1500/1508.html#section2bb).
- Check the robustness of the estimates as a function of noise amplitude by adding random noise to images ahead of fitting. Fitting the signal equation to voxels with noise only (e.g. voxels outside of the brain) is time consuming and give random results. Hence, you can benefit from excluding such voxels by simple masking. Even so, there may still be voxels where the results get weird for real images (outliers), e.g. due to limited SNR, motion or blood flow. Thresholding the resulting PD and T1 maps so that only voxels with reasonable values are shown, can improve the visualization considerably. Beware of outliers when doing statistics. Scaling the PD images, so that CSF gets a value of approximately 1 is recommended.
Follow-up lecture + remaining slides from last week:
- The Bloch equations for time varying
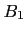 -field and/or spatially varying
-field and/or spatially varying 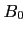 -field. The small tip angle approximation and beyond. Nishimura chapter 6, p. 107-118.
-field. The small tip angle approximation and beyond. Nishimura chapter 6, p. 107-118.
Michelle and Mark present:
- Instrumentation: Magnet (field strength, homogeneity, volume). RF transmitter and receiver system (channels, power), gradient system (strength and slew rate), coils (sensitivity, inhomogeneity, trade-offs). Siting. Nishimura page 48-49. Nishimura chapter 11.
The exercise from last weeks lecture continues. You will need the developed software for the
first report assigment that you can work on now.
Follow-up lecture:
- Spectroscopy localization techniques: Single voxel and
spectroscopic imaging.
Mikkel and Anders present:
- The Bloch equations for temporally varying gradients. Phase rolls, k-space, imaging. Nishimura p. 61-74.
Today's exercise leads up to the analysis of the brain spectroscopy data
acquired. As in the earlier FLASH exercise, initially constructed artificial
signals are later used for validating of software that you write
for analysis of spectra. You will find the spectroscopy review on
CampusNet useful.
This exercise continues over more weeks. For next week: Bring your spectroscopy data from the practical exercise. Note: In matlab A' means the transpose of A, but it also gets complex conjugated!! Use .' if you want to avoid that.
- Write a function, that given the following parameters return a corresponding FID: A frequency shift in ppm, a relaxation time (e.g. 100 ms), the number of samples (e.g. 1024), the time between samples (called the dwell time, e.g. 1 ms).
- Use this function to generate an artificial FID with frequencies at NAA, Cho, Cr and water frequencies (weighted sum). Plot the corresponding spectrum on conventional axis (hint: start with Hz units, then ppm. See slide with both). You choose the amount of each signal, e.g. similar weights.
- Construct a design matrix (model) based on the individual metabolite spectra. Use this to recover the chosen weights.
- Extend the design matrix to accomodate spectral distortions: Non-suppressed water, slowly varying baseline, lipids.
- Modify the function to use a water reference signal as line shape reference. Try the above with a water reference that is slightly off resonance (e.g. resulting if the field changing slightly between metabolite and water reference acquisition.
Try the interactive
FID explorers that will give you a good understanding of the relation between FIDs and spectra(ask if they don't). Try in connection with reading the brain spectroscopy review in the Handouts folder.
The spectroscopy lectures continue (see above).
The student presentation is postponed, and the following presentations are shifted 1 week.
Continue last weeks spectroscopy exercise. Find your spectroscopy data from the practical exercise in the ExerciseData folder. Conversion to a readable format can be tricky and depends much on the scanner used for acquisition. You are not expected to do this yourself.
Spectral modelling. K-space (last week's slides). RF quiz.
Isabelle and Steen present:
- k-space filling techniques for 2D and 3D imaging. Single shot, multi-echo, multi-slice and segmented acquisition techniques. Nishimura chapter 8. Go easily over projection methods and skip artifacts (covered later).
Continue last weeks exercise, and use a design matrix formed from the water reference acquisition to model your acquired spectroscopy data, i.e. construct a metabolite basis from the water reference acquisition, and use this to estimate peak areas of the metabolite peaks relative to water.
Spectroscopy assignment follow-up: The role of derivatives in spectroscopy analysis is discussed (see slides) and the noise amplification that follows from unfiltered T2*-adjustment of the water reference (see Spectroscopy Quantification course note on CampusNet).
Lars introduces BOLD contrast used for fMRI.
fMRI material is available via CampusNet under links: ``Principles of Functional MRI'', in particular chapter 1 by Seong-Gi Kim and Peter Bandettini (who has a good collection of slides on the net).
Anna and Sidsel present:
- Aliasing, ringing, chemical shift artifact, bandwidth per pixel, B0- and/or B1-inhomogeneity effects. Slice cross-talk. Nishimura p. 84-92 (section 5.7.1-2). Nishimura p. 127-134 (section 7-7.1.2).
Continue last weeks spectroscopy exercise. Add derivatives to design matrix as appropriate. Calculate spectra, fits, peak areas and graphs as described in this weeks spectroscopy analysis lecture. This is part of the next assignment.
The last part of spectroscopy analyses: Derivatives in the design matrix (if needed). MRI instrumentation slides. RF quiz follow-up (see Handouts).
Also some follow-up on BOLD fMRI acquisition and the general linear model for analysis.
Wenjun presents fMRI preprocessing,
i.e., data processing steps before design matrix construction. See CampusNet and plenty of material on the web. An example is described in the SPM manual chapter 28, "Auditory fMRI data". Please walk us through this until "model specification", not in detail or in practice, but copying figures into a presentation, for example (similar material from elsewhere is fine too):
http://www.fil.ion.ucl.ac.uk/spm/data/auditory/.
The spectroscopy assignment continues during the exercise.
BOLD imaging follow-up.
Lars presents:
Relaxation mechanisms, e.g. as described in Nishimura page 51, or MR notes at
http://eprints.drcmr.dk/37/ section 5.2. See also "UnderstandingSpectra.pdf" at CampusNet. Also
MR contrast agents and their use (e.g. flow, perfusion, brain inflammation).
The spectroscopy assignment continues during the exercise (final week!). Ask if you are ready for a new assignment.
Lars follows up on k-space filling and artifacts.
After introduction in Skype, start "building" a sequence, including calculation of various sequence parameters (example gradient and RF field strenghts, bandwidths...).
Scanner specifications:
B0 = 3T; Gmax = 40 mT/m; Slew rate = 100T/m sec; B1max = 20 uT
- How fast can we do a 90 degree excitation?
- Consider RF pulse properties for slice selection in relation to this scanner, and brain imaging with 5mm thickness (T2 around 100ms, T2* around 40ms in well-shimmed areas, T1 around 1s in tissue) (consider the trade-offs involved in choosing a pulse wrt. shape and duration).
- For an RF pulse with 1 kHz bandwidth, which gradient is needed to get 5mm slice thickness?
Design an EPI sequence for BOLD imaging, for example, with (3mm)x(3mm) in-plane resolution, 128x128 matrix.
- Which part of k-space do we need to measure, and how densely? (show non-connected grid points)
- How strong does the frequency encoding gradient (aka readout gradient) need to be, approximately?
- How much is the fat/water displacement (mm) in the slice, readout (aka frequency encoding direction), and the phase encoding (blip) direction?
This week, we will meet on Zoom (invitation follows via DTU Inside)! New topic:
Flow effects and angiography: Time-of-flight, black/bright
blood and phase contrast.
Nishimura chapter 10 excluding Fourier
velocity imaging.
Diffusion weighted MRI. See Jones DK, from book "Diffusion MRI", edited by Behrens and
Johansen-Berg. Only page 37 to 48 (sections I-III) are exam relevant, excluding section
III.D "How to estimate the diffusion tensor" and section III.E.3 "Tensor shape(Westin Metrics)". Available as GaussianModelingOfDiffusion.pdf on CampusNet/Handouts.
After follow-up on last week's exercise, it continues in an extended version.
This week, we will meet on Zoom (invitation follows via DTU Inside)! Lars follows up on diffusion weighted imaging. Stjernekollega Pernille Rose Jensen presents:
- Hyperpolarization: A very remarkable, upcoming technique that improves the MR signal by roughly a factor 10000(!) in specific situations. Pernille talks about methods and applications of hyperpolarized imaging and spectroscopy, which is a main focus of the DTU MR group, and collaborating groups at e.g. Rigshospitalet and Skejby (and many other outside of Denmark).
- You will also hear about the MR facilities on the 1st floor of building 349 and elsewhere,
- ....and briefly hear about Pernille's upcoming 3-week course, 22508 (formerly 31552, Practical NMR spectroscopy: Making reactions in (bio)chemistry visible).
You will also briefly hear about the fall course Advanced Magnetic Resonance Imaging taught mostly by Axel Thielscher and myself (see how it fits in the MRI education). In this course we dig deeper into measurement design, ultra-highfield MRI, relaxation theory, RF aspects, fMRI and hyperpolarized MR. More reading and discussion, less reports.
There is no exercise this week, but classroom activities will continue after 3 o'clock.
Follow-up on whatever else needs to be rounded off (questions and topic suggestions are welcome, and not necessarily needed in advance).




 Print Version
Print Version